What Is the Molar Mass of Ammonium Sulfide
Molar Mass Molecular Weight and Elemental Composition Calculator Enter a chemical formula to calculate its molar mass and elemental composition. What is the molar mass of ammonium sulfate.

Molar Mass Of Nh4 2s Ammonium Sulfide Note Final Answer Should Be 68 13 Youtube
For example the molar mass of Ammonium Sulfate is 13214 grams so the weight in grams of 100 ammonium sulfate would be 13214 grams.
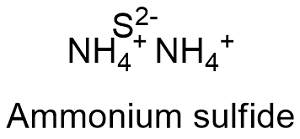
. 13214 gmol Melting Point. How do you calculate molar mass. Formula Calculate Formatted Formula NH 4 2 S Empirical Formula H 8 N 2 S Molar Mass 6814.
About Ammonium hydrogen sulfide. Online molar mass calculator molecular weight calculator MW for a chemical formula with letter case correction and multi-section formula Molar Mass of Ammonium Sulfite Molecular Weight of NH 4 2 SO 3. Ammonium Sulfate ACS Reagent.
How many CL are in a L. The mass of 00914 mol ammonium sulfate is 121 g rounded to three significant figures due to 00914 mol. Molar Mass of Ammonium Sulfide Molecular Weight of NH 4 2 S.
The molar mass of lead IV sulfate is 39932gmol1. The best way to calculate molar mass of a compound is by counting the number of atoms of a type present in it and by multiplying the number with the molecular mass of that atom. The molecular weight of ammonium sulfate is 13214 gmol.
Colorlesswhite solid Physical State. Ammonium Sulfate 100 Grade. Ammonium sulfide is a yellow crystalline sand-like solid that is commonly seen in an aqueous solution.
Ammonium Sulfate NH42SO4 - Ammonium sulfate appears as a white crystalline solid. Explanation of how to find the molar mass of NH42SO4. Molar mass of NH42SO4 13213952 gmol.
Ammonium hydrogen sulfide weighs 117 gram per cubic centimeter or 1 170 kilogram per cubic meter ie. NH_4_2SO_4 an important synthetic fertilizer. Density of ammonium hydrogen sulfide is equal to 1 170 kgm³In Imperial or US customary measurement system the density is equal to 73041 pound per cubic foot lbft³ or 06763 ounce per cubic inch ozinch³.
Ammonium sulfate is an inorganic sulfate salt obtained by reaction of sulfuric acid with two equivalents of ammonia. Treating ammonium hydroxide with an excess of hydrogen sulfide ammonium hydrosulfide NH 4 HS is formed which with further treatment with the. A common request on this site is to convert grams to moles.
Online molar mass calculator molecular weight calculator MW for a chemical formula with letter case correction and multi-section formula. It is widely used as a fertilizer for soil. The molar mass of ammonium sulfate is 13213952 gmol.
When calculating molecular weight of a chemical compound it tells us how many grams are in one mole of that substance. When calculating molecular weight of a chemical compound it tells us how many grams are in one mole of that substance. To complete this calculation you.
The answer is B 10-2. A high-melting decomposes above 280 white solid which is very soluble in water 706 g100 g water at 0. Ammonium Sulfide NH 4 2 S.
Molar mass of NaCl is 58443 how many grams is 5 mole NaCl. Explanation of how to find the molar mass of NH42S. 1038 g100 g water at 100 it is widely used as a fertilizer for alkaline soils.
Ammonium sulfate Molar mass. Finding molar mass starts with units of grams per mole gmol. Grams mole molar mass.
5 rows Ammonium Sulfate molecular weight. Chemistry questions and answers. An atom of an element has a mass of 663 times 10-23 g.
Ammonium sulfideA few things to consider when finding the molar mass for NH42S- make sure you have. Ammonium sulfide formula is NH4 2 S. For ammonium sulfate this is N H 42SO4 2883264 132 gmol.
To Learn more about the Preparation Properties Uses and FAQs of ammonium sulfate Visit BYJUS for more content. By definition the gram weight on earth of a mole of any compound is the molar mass of that compound. The total number of oxygen atoms in 100 g of CaCO_3 MM 1000 gmol is How many moles of iron atoms are contained in 609 g of iron.
Molar mass of NH42SO4 2 1401 8 10079 3206 4 159993g 2802 80632 3206 639972 g 13214 g Mola View the full answer Transcribed image text. The mass and molarity of chemical compounds can be calculated based on the molar mass of the compound. Molar mass of NH42S is 681419 gmol.
It has a distinct rotten egg odour and is utilized in the photography and textile industries. Ammonium sulfateA few things to consider when finding the molar mass for NH42SO4- make sure you h. 5 to 6 Solubility Information.
The mass in grams of a compound is equal to its molarity in moles multiply its molar mass. They are highly flammable and the ammonium sulfide molecular formula is represented as follows NH4 2 S. ACS Reagent CAS Number.
The formula weight is simply the weight in atomic mass units of all the atoms in a given formula. Molartext masstext oftext NH_4_2SO_4 2 times N 8 times H 1 times S 4 times O 2 times 140067 8 times 100784 1 times 32065 4 times 159994 13213872gmol - 1. Thus 00914 moles has a mass of 00914 mol 132 gmol 1206g.
Ammonium sulfide also known as the stink bomb is a toxic chemical and has a very strong and unpleasant odour.

Sa Q39 Nh4s How To Find The Molar Mass M Of Ammonium Sulfide Youtube

Calculate The Molar Mass Of Nh4 2so4 Ammonium Sulfate Molar Mass Practice Youtube
No comments for "What Is the Molar Mass of Ammonium Sulfide"
Post a Comment